Treatment of diabetes by the medical community used to be straight-forward. Insulin (usually pig-derived) was administered and the patients’ condition improved (usually) but was by no means cured.
Insulin was discovered in the early 1920s by Canadian physician Frederick Banting and medical student Charles Best. Development and extraction of the hormone took place through the collaboration with John MacLeod and chemist James Bertram Collip at the University of Toronto. Once purified, the extract was injected into a diabetic dog, the dog recovered, and the possibility of new life for type I diabetic patients was born.
Since those early years, many drugs for treatment of diabetes have been developed. The incidence of diabetes has skyrocketed, fueling the need and a market for those diabetes drugs. The list of drugs developed is long and getting more confusing every year. In the hopes of relieving some of the confusion, this list of drugs and their actions is presented.
Metformin has long been recommended as first-line therapy (after diet and lifestyle change which are mentioned but not really described in any degree of detail in most medical offices, other than “stop drinking sodas” and “do some exercise”). Metformin restores leptin sensitivity in obese nondiabetic patients. A study of 10 patients published in 2001 showed that metformin increased the secretion of glucagon (GLP-1) but had no effect on Leptin levels in obese non-diabetic men.[1] Metformin reverses fatty liver disease in both mice[2] and humans.[3] The mechanism of action is pretty much unknown. Nevertheless, metformin is the first line drug in current recommendations for T2Dm therapy.[4]
On a further note, there is good evidence that lifestyle modification of the right sort and Metformin have about the same effects in lowering blood sugar and restoring insulin sensitivity, at least in Asian Indians.[5] So if we were really serious about eating a low-glycemic diet and embarking on a course of daily exercise and forgiveness of those who we think have wronged us, it would probably have the same glucose-lowering effect as Meformin – and would be a whole lot healthier for our entire being as well. But that is material for another article.
 GLP-1 receptor agonists – (GLP-1 RA) slow gastric emptying, thus increasing the feeling of satiety (fullness). These drugs are administered by injection, they increase insulin secretion in those who can still produce insulin, and they suppress glucagon production. Glucagon induces break-down of fat to provide calories to the body. Its suppression would be associated with weight gain, however the GLP-1 agonist appears to be an adequate substitute, because these drugs are associated with weight loss as well as reduction in blood pressure. One downside is that they do result in significant nausea in 30% of patients. Another substantial downside is the risk of thyroid medullary cancer. This notification appears as a black box warning on all the GLP-1 RA drugs, Exenatide (Byetta®), Exenatide ER (Bydureon®), Liraglutide (Victoza®), Lixisenatide (Luxymia®), Dulaglutide (Trulicity®), Semaglutide (Ozempic®). Yet another downside is that all of them may be associated with increased risk of kidney failure.
GLP-1 receptor agonists – (GLP-1 RA) slow gastric emptying, thus increasing the feeling of satiety (fullness). These drugs are administered by injection, they increase insulin secretion in those who can still produce insulin, and they suppress glucagon production. Glucagon induces break-down of fat to provide calories to the body. Its suppression would be associated with weight gain, however the GLP-1 agonist appears to be an adequate substitute, because these drugs are associated with weight loss as well as reduction in blood pressure. One downside is that they do result in significant nausea in 30% of patients. Another substantial downside is the risk of thyroid medullary cancer. This notification appears as a black box warning on all the GLP-1 RA drugs, Exenatide (Byetta®), Exenatide ER (Bydureon®), Liraglutide (Victoza®), Lixisenatide (Luxymia®), Dulaglutide (Trulicity®), Semaglutide (Ozempic®). Yet another downside is that all of them may be associated with increased risk of kidney failure.
The GLP-1 receptor agonists are generally more expensive than other agents. GLP-1 is one of the incretin hormones whose function is to regulate insulin and glucagon release, gastric emptying and caloric intake. There are receptors for GLP-1 in the hypothalamus as well as in the pancreas. GLP-1 controls the reward effect induced by alcohol, amphetamines and cocaine. GLP-1 receptors are expressed in areas significantly associated with reward regulation.[6] Is it any wonder that we often eat “emotionally” – when we feel bad, it is nice to get a hit of dopamine and feel better. Byetta® derived from Gila monster venom, was approved in 2005 by the FDA for treatment of type 2 diabetes.[7] GLP-1 levels are increased by ingestion of green plant membranes on a regular basis.[8], [9] GLP-1 is secreted by the L cells of the distal gut during food intake. Ghrelin is produced by cells of the stomach in response to hunger and appears to stimulate the secretion of GLP-1.[10] GLP-1 in turn suppresses ghrelin secretion thus suppressing the sensation of hunger and need to ingest food.[11]
These GLP-1 receptor agonists might be effective for those who eat emotionally – if they don’t mind risking kidney failure or thyroid cancer in the process.
DPP-4 inhibitors – (Gliptins) – work by blocking the action of DPP-4, an enzyme which breaks down GI hormones called incretins (including ghrelin) – these stimulate the production of insulin in response to a meal. Adverse effects include nausea, diarrhea, abdominal pain, flu-like symptoms, pancreatitis. The good side of gliptins is that they suppress inflammation, thus reducing oxidative stress on the entire system.[12] This class of drugs can be useful in patients who already have fatty liver and signs of heart failure – except for saxagliptin (Onglyza®) which is associated with increased incidence of heart failure. Anther potential downside is that Kidney function may be impaired by some of the DPP-4 inhibitors including alogliptin Nesina®, saxagliptin Onglyze® and sitagliptin Januvia®. Linagliptin Tradjenta® does not appear to impair renal function.
Alpha glucosidase inhibitors – AGis – work by competitive and reversible inhibition of intestinal enzymes that break down carbohydrates into smaller molecules like glucose for easier absorption. Miglitol (Glyset®) and acarbose (Precose®) are two examples. Significant cardiovascular benefits have been observed.[13] These drugs appear to improve endothelial function – (the cells lining the inside of the blood vessels), thus reducing the chance of developing vascular disease (arteriosclerosis) and lessening the chance of stroke, heart attack or limb amputation.
PPAR gamma agonists like the thiazolidinediones and metformin stimulate adiponectin levels in obese mice and insulin-resistant humans and increase their insulin sensitivity.[14], [15], [16] Rosiglitazone (Avandia®) and pioglitazone (Actos®) are two examples of thiazolidinediones. Troglitazone (Rezulin® was removed from the US market due to liver toxicity). Metformin does have a different spectrum of activity from the other thiazolidinediones.[17]
The PPAR superfamily (peroxisome proliferator-activated receptor) – alpha, gamma and beta/delta – are transcription factors that belong to the Superfamily of nuclear receptors. Other members of this family include retinoic acid, estrogen, thyroid, vitamin D, and glucocorticoid receptors, and several other proteins involved in xenobiotic (foreign compound) metabolism.[18]
- Activation of PPAR-α reduces triglyceride level and is involved in regulation of energy homeostasis. Activation of PPAR-γ causes insulin sensitization and enhances glucose metabolism. PPAR-alpha agonists induce cholesterol removal from macrophages.
- PPAR-γ regulates energy storage, whereas PPAR-α is expressed predominantly in the liver, and to a lesser extent, in muscle, in the heart, and in bone and PPAR-δ present ubiquitously expressed in whole body regulate energy expenditure; expression of PPAR-γ in endothelial cells, vascular smooth muscle cells. PPAR-γ is further subdivided in four isoforms expressed in different tissues of the body and having presumably slight different functions.
- γ1 - expressed in virtually all tissues, including heart, muscle, colon, kidney, pancreas, and spleen.
- γ2 - expressed mainly in adipose tissue (30 amino acids longer).
- γ3 - expressed in macrophages, large intestine, and white adipose tissue.
- γ4 - expressed in endothelial cells.
- activation of PPAR-β/δ enhances fatty acids metabolism.”[19]
Both PPAR-alpha and PPAR-gamma receptors are present in endothelial smooth muscle cells and macrophages.[20] Rats have higher expression of PPAR-alpha than humans, so rat studies may not accurately reflect the lipid benefits in humans.[21]
Both metformin and rosiglitazone are PPAR agonists. However, as you can see from the chart below, for patients with hepatic steatosis (fatty liver), Metformin would NOT be first choice diabetes drug. Rosiglitazone would be preferable.
Metformin | Rosiglitzaone |
Decrease Hgb A1C, insulin and free fatty acid concentration. | Decrease Hgb A1C, insulin and free fatty acid concentration. |
Decrease body weight | No decrease in body weight |
No change in liver fat (perhaps because humans do not have high expression of PPAR-alpha) | Decrease liver fat |
Some increase in hepatic insulin sensitivity, some increase in liver/peripheral glucose uptake | Marked increase in hepatic insulin sensitivity, liver/peripheral glucose uptake |
Serum adiponectin unchanged | Serum adiponectin increase 123% |
No increased expression of peroxisome proliferator-activated receptor-gamma, adiponectin, and lipoprotein lipase in adipose tissue | Increased expression of peroxisome proliferator-activated receptor-gamma, adiponectin, and lipoprotein lipase in adipose tissue |
No decrease in liver fat, increases insulin clearance | Decreases liver fat, increases insulin clearance |
SGLT2 inhibitors act by increasing renal excretion of glucose.[22] The process is a little like increasing the size of the bathtub drain without actually decreasing the flow of water into the tub. If the water is dirty, the tub will not magically be filled with clean water – just perhaps less dirty water. There are three SGLT2 inhibitors currently on the market – The glyflozin drugs include: canagliflozin (Invokana®), empagliflozin (Jardiance®), Dapagliflozina (Farxiga®) and Ertugliflozin (Steglatro®). They are all taken by mouth. The downsides:
- some of these drugs are also associated with increased incidence of renal failure, bladder cancer and bone fractures. One has to wonder whether these “side effects” might have something to do with the excessive levels of sugar present in the urine 24 hours a day, and the potential for chronic systemic acidosis, i.e. systemic inflammation? It would seem preferable to control the glucose levels upstream.
- The biggest downside of these drugs is the risk of euglycemic diabetic ketoacidosis.[23] Patients with euglycemic ketoacidosis have a “normal” blood glucose, but are severely acidotic and very ill.
MRA inhibitors (antimineralocorticoid) are used for T2Dm patients who are already in renal failure, to avoid increasing serum potassium levels.[24] Some examples include nonsteroidal MRA, apararenone (not available in the USA) esaxerenone (Minnebro™) and finerenone (currently in phase 3 trials). All these have affinity for glucocorticoid receptors as well as mineralocorticoid, thus resulting in side effect of things like gynecomastia, impotence, low libido.
New forms of Insulin therapy –
Inhaled insulin - Afrezza® - is an insulin powder for inhalation. It has a rapid onset of action (15 minutes). However it is not recommended for patients with asthma or other chronic lung disease. This form of insulin may be associated with fluid retention or heart failure, especially when used together with the thiazolidinediones (PPAR agonists) which also have those same side effects. For more information, see this article from the New England Journal of Medicine.[25]
Injectable concentrated insulins
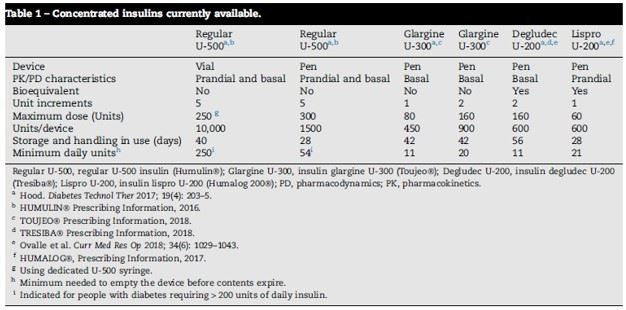
Reproduced from Schloot, N. C., et al. "Concentrated Insulins in Current Clinical Practice."
Diabetes research and clinical practice (2018).
 Insulin glargine U-100 (Tresiba®) and U-300 (Basaglar®, Toujeo®) – this is a so-called “basal insulin” intended to be a baseline dose upon which to layer other diabetes medications. It is 3 times as concentrated as the original long acting insulin and is distributed as a multi-dose pen. There are two available pens, 450 units and 900 units, each allowing a maximum dose of 80 units per injection (with the 450 unit pen) or 160 units (with the 900 unit pen). These formulations are stable at room temperature for 42 days, seven weeks. Compared with Insulin glargine U-100 (Lantus®) there was less hypoglycemia (clinically obvious low blood sugar).
Insulin glargine U-100 (Tresiba®) and U-300 (Basaglar®, Toujeo®) – this is a so-called “basal insulin” intended to be a baseline dose upon which to layer other diabetes medications. It is 3 times as concentrated as the original long acting insulin and is distributed as a multi-dose pen. There are two available pens, 450 units and 900 units, each allowing a maximum dose of 80 units per injection (with the 450 unit pen) or 160 units (with the 900 unit pen). These formulations are stable at room temperature for 42 days, seven weeks. Compared with Insulin glargine U-100 (Lantus®) there was less hypoglycemia (clinically obvious low blood sugar).
Human regular U-500 insulin – Reg-U500 (Humulin® R U-500) – this humaninsulin analog comes in a 20 ml vial stable at room temperature for 40 days (almost 7 weeks). Up to 250 units of insulin may be administered at one time using a dedicated U-500 syringe. Reg-U-500 also comes in a multi-dose pen, which is stable at room temperature for four weeks only.
Insulin degludec U-100 and U-200 – Deg-U200 (Tresiba®) – ultra long-acting insulin analog available in 3 ml pens delivering up to 160 units per dose. Degludec U-100 and U-200 are bioequivalent, meaning that a single dose of U-200 delivers twice the effect on blood sugar as the same volume dose of U-100. Deg-U200 is stable at room temperature for 56 days, almost 2 months. Other ingredients in this formulation of insulin include glycerol, metacresol, phenol and zinc acetate.
Insulin Lispro U-100 and U-200 (Humalog®) – is a short-acting insulin analog intended to be administered after a meal. , and can deliver up to 60 units per injection. It is stable at room temperature for 28 days. The multi-dose pens deliver Lispro insulin in 1 unit increments. Lispro-200 is stable at room temperature for 28 days.
Where does insulin come from?
All the currently available insulins are actually analogs, similar but not identical to human insulin. The analogs are manufactured with the cooperation of genetically-modified bacteria and then purified before packaging. None is identical to human insulin (which cannot be patented, since it is a natural product). Pig insulin differs by one amino acid, beef insulin by 3 amino acids, Lispro by 2 amino acids.

[1] Mannucci, Edoardo, et al. "Effect of metfoin on glucagon-like peptide 1 (GLP-1) and leptin levels in obese nondiabetic subjects." Diabetes Care 24.3 (2001): 489-494.
[2] Lin, Hui Zhi, et al. "Metformin reverses fatty liver disease in obese, leptin-deficient mice." Nature medicine 6.9 (2000): 998.
[3] Nar, Aslı, and O. L. C. A. Y. Gedik. "The effect of metformin on leptin in obese patients with type 2 diabetes mellitus and nonalcoholic fatty liver disease." Acta Diabetologica 46.2 (2009): 113-118.
[4] Foretz, Marc, et al. "Metformin: from mechanisms of action to therapies." Cell metabolism 20.6 (2014): 953-966.
[5] Ramachandran, Ambady, et al. "The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1)." Diabetologia 49.2 (2006): 289-297.
[6] Engel, Jörgen A., and Elisabet Jerlhag. "Role of appetite-regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy." CNS drugs 28.10 (2014): 875-886.
[7] Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Journal of Biological Chemistry. 1992;267(11):7402-7405.
[8] Montelius, Caroline, et al. "Body weight loss, reduced urge for palatable food and increased release of GLP-1 through daily supplementation with green-plant membranes for three months in overweight women." Appetite 81 (2014): 295-304.
[9] Stenblom, Eva-Lena, et al. "Supplementation by thylakoids to a high carbohydrate meal decreases feelings of hunger, elevates CCK levels and prevents postprandial hypoglycaemia in overweight women." Appetite 68 (2013): 118-123.
[10] Gagnon, Jeffrey, et al. "Ghrelin is a novel regulator of GLP-1 secretion." Diabetes 64.5 (2015): 1513-1521.
[11] Montelius, Caroline, et al. "Body weight loss, reduced urge for palatable food and increased release of GLP-1 through daily supplementation with green-plant membranes for three months in overweight women." Appetite 81 (2014): 295-304.
[12] Wang, Xiaoyu, et al. "Gliptins suppress inflammatory macrophage activation to mitigate inflammation, fibrosis, oxidative stress, and vascular dysfunction in models of nonalcoholic steatohepatitis and liver fibrosis." Antioxidants & redox signaling 28.2 (2018): 87-109.
[13] Standl, Eberhard, and Oliver Schnell. "Alpha-glucosidase inhibitors 2012–cardiovascular considerations and trial evaluation." Diabetes and Vascular Disease Research 9.3 (2012): 163-169.
[14] Sharabi, Yehonatan, et al. "Effect of PPAR-γ agonist on adiponectin levels in the metabolic syndrome: lessons from the high fructose fed rat model." American journal of hypertension 20.2 (2007): 206-210.
Am J Hypertens. 2007 Feb;20(2):206-10.
[15] Hauner, Hans. "The mode of action of thiazolidinediones." Diabetes/metabolism research and reviews 18.S2 (2002): S10-S15.
[16] Elia, Evelin M., et al. "Link between metformin and the peroxisome proliferator-activated receptor γ pathway in the uterine tissue of hyperandrogenized prepubertal mice." Fertility and sterility 95.8 (2011): 2534-2537.
[17] Tiikkainen, Mirja, et al. "Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes." Diabetes 53.8 (2004): 2169-2176.
[18] ibid
[19] Tyagi, Sandeep, et al. "The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases." Journal of advanced pharmaceutical technology & research 2.4 (2011): 236.
[20] Chinetti, Giulia, et al. "PPAR-α and PPAR-γ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway." Nature medicine 7.1 (2001): 53.
[21] Haluzik, M. M., and M. Haluzik. "PPAR-alpha and insulin sensitivity." Physiological research 55.2 (2006): 115.
[22] Chao, Edward C., and Robert R. Henry. "SGLT2 inhibition—a novel strategy for diabetes treatment." Nature reviews drug discovery 9.7 (2010): 551.
[23] Goldenberg, Ronald M., et al. "SGLT2 inhibitor–associated diabetic ketoacidosis: clinical review and recommendations for prevention and diagnosis." Clinical Therapeutics 38.12 (2016): 2654-2664.
[24] Capelli, Irene, et al. "New mineralocorticoid receptor antagonists: update on their use in chronic kidney disease and heart failure." Journal of nephrology (2019): 1-12.
[25] McMahon, Graham T., and Ronald A. Arky. "Inhaled insulin for diabetes mellitus." New England Journal of Medicine 356.5 (2007): 497-502.

